Embarking on a captivating exploration of “one less than an octet,” this article delves into the fascinating realm of chemistry, where elements defy the norm and forge unique bonds. Brace yourself for a journey that unveils the reasons behind this intriguing phenomenon.
Delving into the intricacies of the octet rule, we’ll uncover the elements that adhere to this chemical principle and those that dare to deviate. Join us as we unravel the secrets of these exceptional elements and the remarkable compounds they form.
Octet Rule: One Less Than An Octet
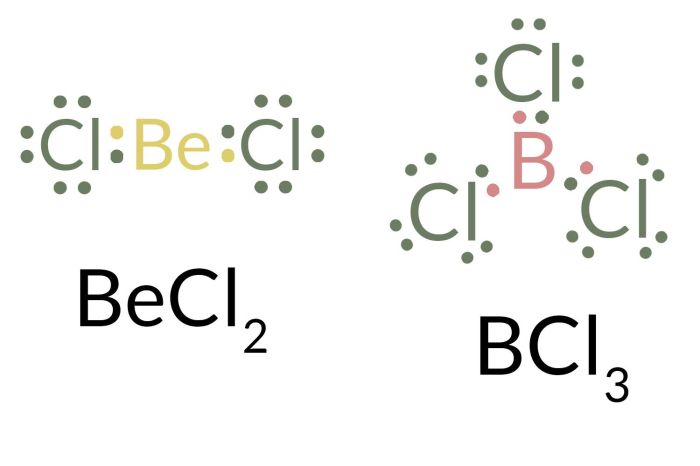
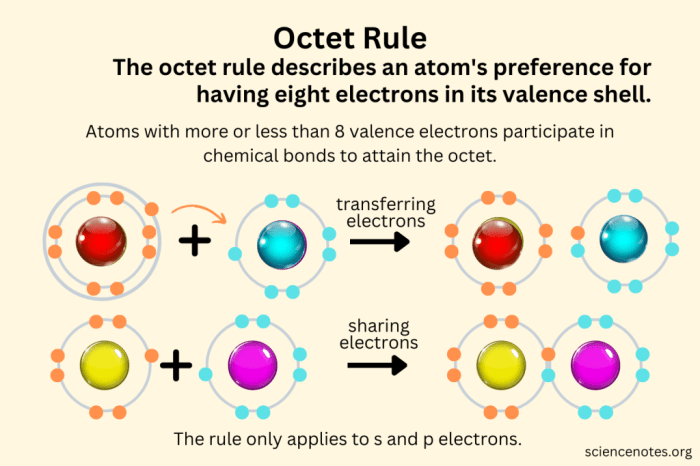
The octet rule is a chemical concept that states that atoms tend to form chemical bonds in such a way that they have eight valence electrons in their outermost electron shell. This rule applies to most elements, particularly those in the second period of the periodic table.The
octet rule is based on the observation that atoms with eight valence electrons are particularly stable. This stability is due to the fact that eight valence electrons form a complete electron shell, which is the most stable electron configuration for an atom.There
are a number of elements that follow the octet rule. These elements include the noble gases, which have eight valence electrons and are therefore very stable. Other elements that follow the octet rule include the alkali metals, which have one valence electron and tend to form bonds with elements that have seven valence electrons, and the halogens, which have seven valence electrons and tend to form bonds with elements that have one valence electron.There
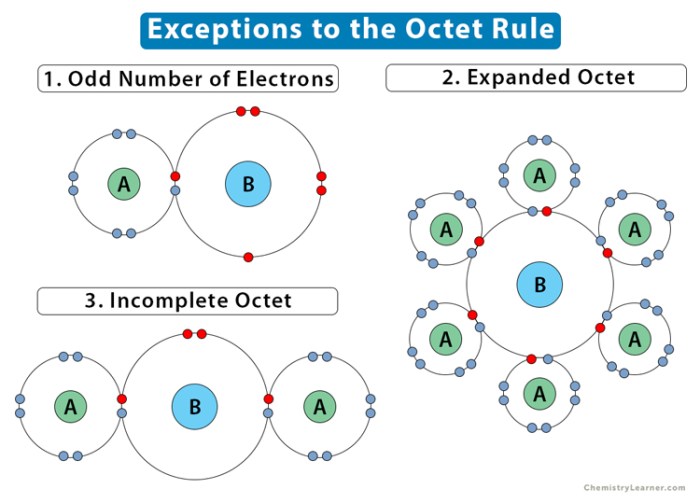
are some exceptions to the octet rule. These exceptions include elements that have less than eight valence electrons, such as hydrogen and helium, and elements that have more than eight valence electrons, such as phosphorus and sulfur. These elements can still form stable compounds, but they do not always follow the octet rule.
“One Less than an Octet”
In chemistry, the “one less than an octet” rule refers to the observation that some elements tend to form stable compounds with one less valence electron than the noble gas configuration with eight valence electrons (an octet).
Elements that typically exhibit this behavior include:
- Boron (B)
- Aluminum (Al)
- Gallium (Ga)
- Indium (In)
- Thallium (Tl)
These elements belong to Group 13 of the periodic table and are known as the boron group elements.
Reasons for One Less than an Octet
The reason why these elements have one less than an octet in their stable compounds is due to their electronic configuration. Boron group elements have three valence electrons in their outermost shell, and they achieve stability by forming covalent bonds with other atoms to complete their valence shell with eight electrons.
However, in certain compounds, these elements can form stable bonds with only six valence electrons. This is because the empty p-orbital in their valence shell can accommodate an additional electron, resulting in a stable configuration with seven valence electrons.
Bonding in Compounds with One Less than an Octet
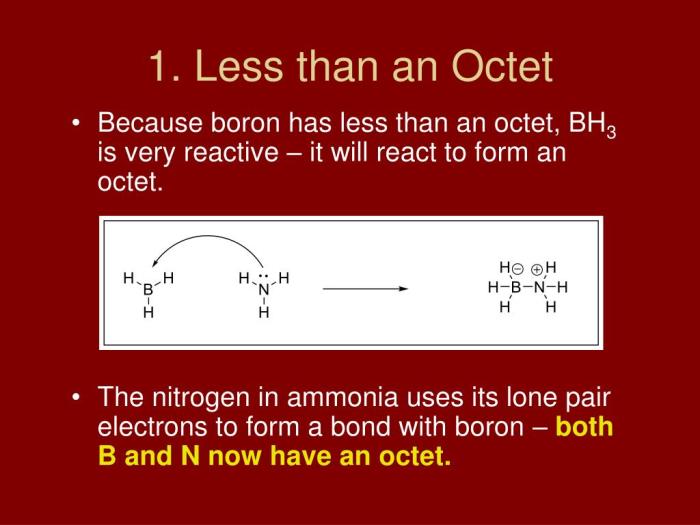
Elements with one less than an octet in their valence shell tend to form covalent bonds to achieve a stable electron configuration.
One common type of bond formed by these elements is the two-electron bond. In this type of bond, each atom contributes one electron to form a shared pair of electrons. This results in a covalent bond between the two atoms.
Examples of Compounds with One Less than an Octet
- Boron trifluoride (BF3) : Boron has three valence electrons, and each fluorine atom has seven valence electrons. In BF 3, each boron atom forms three covalent bonds with the fluorine atoms, sharing one electron with each fluorine atom. This results in a stable octet configuration for each atom.
- Carbon monoxide (CO): Carbon has four valence electrons, and oxygen has six valence electrons. In CO, the carbon atom forms a triple bond with the oxygen atom, sharing three pairs of electrons. This results in a stable octet configuration for both atoms.
- Nitrogen monoxide (NO): Nitrogen has five valence electrons, and oxygen has six valence electrons. In NO, the nitrogen atom forms a double bond with the oxygen atom, sharing two pairs of electrons. This results in a stable octet configuration for the nitrogen atom and a seven-electron configuration for the oxygen atom.
One less than an octet is a chemical concept that refers to atoms with seven valence electrons. For those who need a refresher on basic arithmetic, check out this helpful guide on How To Sum In Excel . Returning to our chemical topic, one less than an octet is a key factor in determining the reactivity of elements.
These compounds are generally stable and have relatively high bond strengths. However, they can be reactive with other compounds, especially those that can donate or accept electrons.
Reactivity of Compounds with One Less than an Octet
Compounds with one less than an octet are highly reactive due to their unstable electron configuration. They readily undergo reactions to achieve a stable octet configuration.
Types of Reactions
Compounds with one less than an octet can undergo various types of reactions, including:
- Ionic bonding:They can form ionic bonds with elements that have a high electronegativity, such as halogens or oxygen.
- Covalent bonding:They can form covalent bonds with other atoms or molecules to share electrons and achieve a stable octet.
- Redox reactions:They can act as reducing agents, transferring electrons to other atoms or molecules to gain electrons and achieve a stable octet.
Examples of Reactions
Here are some examples of reactions involving compounds with one less than an octet:
- Sodium (Na) reacts with chlorine (Cl) to form sodium chloride (NaCl):2 Na + Cl 2→ 2 NaCl
- Hydrogen (H) reacts with oxygen (O) to form water (H2O): 2 H + O 2→ 2 H 2O
- Magnesium (Mg) reacts with hydrochloric acid (HCl) to form magnesium chloride (MgCl2) and hydrogen gas (H 2): Mg + 2 HCl → MgCl 2+ H 2
These reactions illustrate the high reactivity of compounds with one less than an octet and their tendency to undergo reactions to achieve a stable electron configuration.
FAQs
What is the octet rule?
The octet rule states that atoms tend to gain or lose electrons until they have eight valence electrons, resulting in a stable electron configuration.
Why do some elements have one less than an octet?
Certain elements, such as boron and aluminum, have only three valence electrons and cannot achieve a stable octet. Therefore, they form compounds with one less than an octet.
What types of bonds do elements with one less than an octet form?
Elements with one less than an octet typically form covalent bonds, sharing electrons with other atoms to achieve stability.